Organic Reactions® provides a compilation of an authoritative summary of a preparatively useful organic reaction from the primary literature. Practitioners interested in executing such a reaction (or simply learning about the features, advantages, and limitations of this process) thus have a valuable resource to guide their experimentation. Abstracting services, such Chemical Abstracts and Beilstein, allow for the practitioner to locate all of the literature on the subject, but without providing insight into the value of any particular reference.
Organic Reactions® chapters constitute a distillation of this avalanche of information into the knowledge needed to correctly implement a reaction and are much more than a surfeit of primary references. This capacity, namely to provide focused, scholarly, and comprehensive overviews of a given transformation, that Organic Reactions takes on even greater significance for the practice of chemical experimentation in the 21st century. The suitability of a given reaction for an unknown application is best judged from the informed vantage point provided by precedent and guidelines offered by a knowledgeable author as provide in Organic Reactions.
Complete list of published chapters may be found at: https://OrganicReactions.org/published-chapters/.
Visit the Organic Reactions® web page for more information.
Organic Reactions® regularly financially supports Organic Division programs and they cosponsor the Roger Adams Award which recognizes and encourage outstanding contributions to research in organic chemistry defined in its broadest sense. The award is presented biennially in odd-numbered years at the National Organic Chemistry Symposium (NOS). A video of the 2023 Award presentation including some of the history of Organic Reactions is available here.
Organic Reactions – Officers
Dr. P. Andrew Evans, Editor-in-Chief
Dr. Steven Weinreb, Executive Editor
Dr. Barry Snider, Secretary
Latest from Organic Reactions
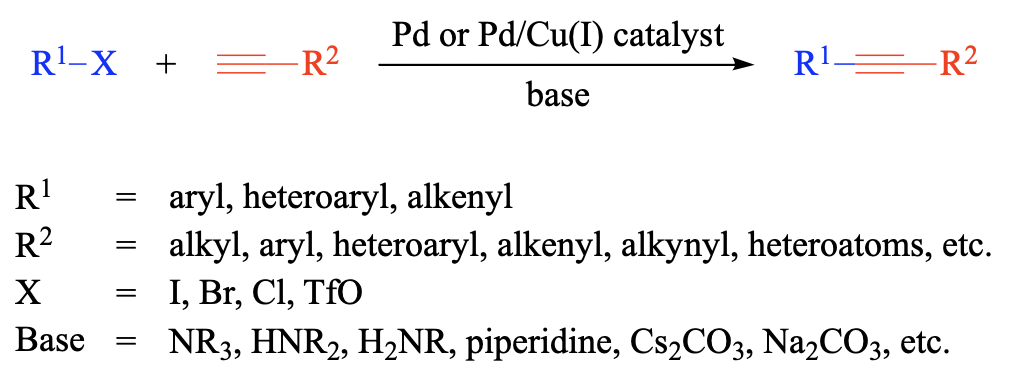
Conjugated alkynes are valuable intermediates in the synthesis of natural products, agrochemicals, pharmaceuticals, fine chemicals, and organic molecular materials. Since its introduction in 1975, the palladium-catalyzed Sonogashira reaction has become [...]
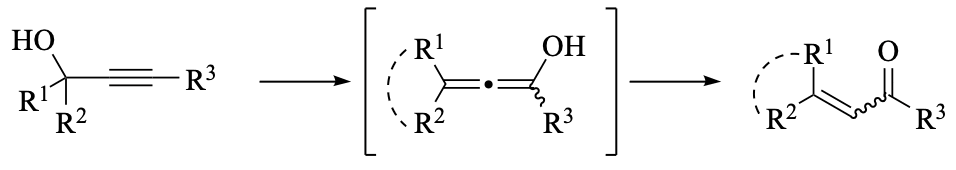
The Meyer–Schuster rearrangement corresponds to a formal 1,3-shift of a propargylic alcohol to afford the corresponding α,β-unsaturated carbonyl compound via tautomerization of an allenol intermediate. The original acidic and harsh [...]
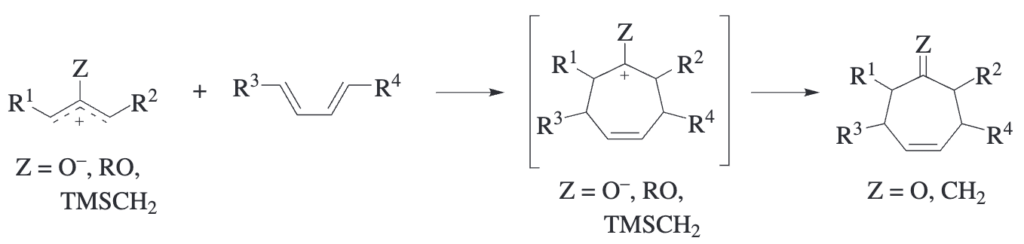
The reaction of allylic cations with 1,3-dienes is formally equivalent to the Diels–Alder reaction but leads to seven-membered rings. An allylic cation contains 2 pi electrons, and is thus precisely [...]
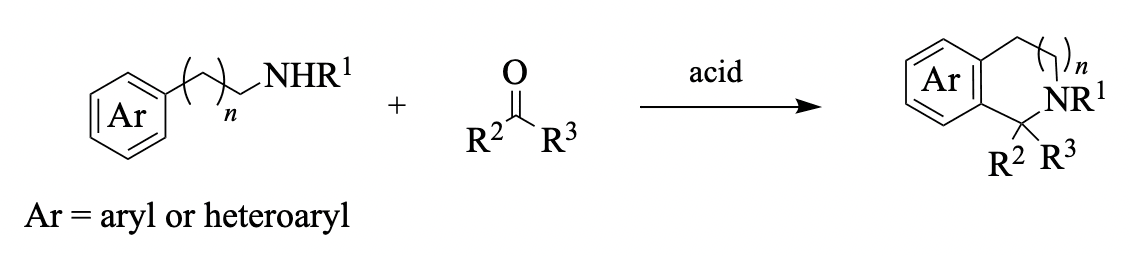
Enantioselective Pictet–Spengler reactions are promoted or catalyzed by small chiral molecules, including Brønsted acids, Lewis acids, and hydrogen-bond donors (e.g., thiourea compounds). While tryptamines and β-phenethylamines are commonly used in [...]
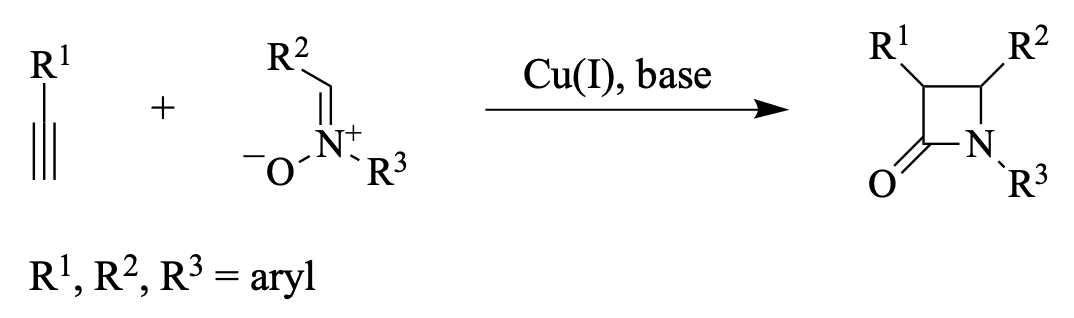
Among the methods of synthesizing the β-lactam ring, the copper(I)-mediated reaction between a nitrone and a terminal alkyne in the presence of an organic base is a remarkably simple and [...]
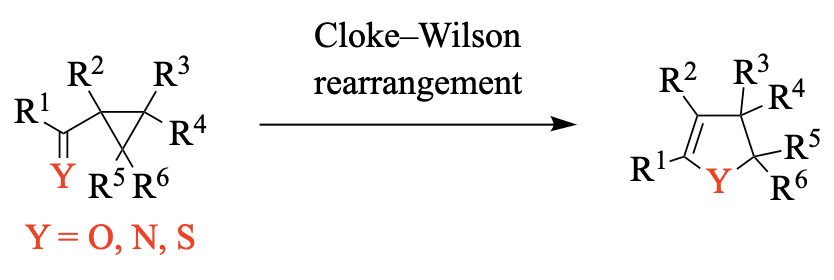
The Cloke–Wilson rearrangement is a reaction that converts cyclopropanes bearing a carbonyl, thiocarbonyl, or imino group into dihydrofurans, dihydrothiophenes, or dihydropyrroles, respectively, in a transformation that is generally driven by [...]
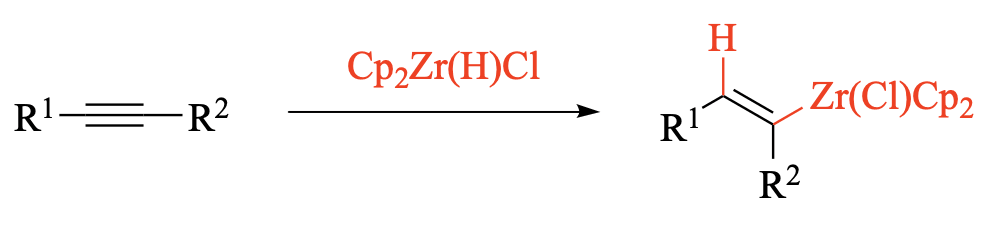
Since the first preparation of organozirconocenes from alkenes and alkynes in 1972, hydrozirconation has become one of the most commonly used stoichiometric methods to convert readily available starting materials into [...]
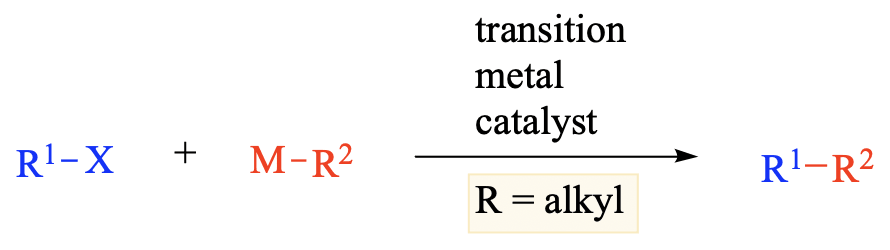
The cross-coupling reaction of alkyl halides with alkyl metal reagents is a straightforward synthetic method to construct saturated carbon frameworks. Although the development of alkyl–alkyl cross-coupling reactions has lagged behind [...]
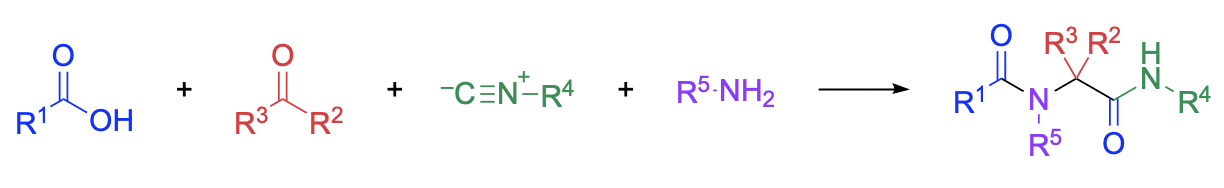
The classic Ugi reaction is a four-component coupling process involving a carboxylic acid, a carbonyl compound, an amine, and an isocyanide, resulting in an N-acylamino acid amide product. Modified Ugi [...]